FC FRANCE SAS is a CDMO
(Contract Development and Manufacturing Organization)
Specialised in:

The research

The development

The adjustment

The manufacture

The purchase

The sale

The packaging

The storage

The marketing
of veterinary pharmaceutical products, as well as all the products in relations with the medical field, in all forms.
ANSES / GMP Approval: V 0594/81
897 891 370 – RCS Pontoise
Intra-community VAT: FR30897891370
ACTIVITIES
MEDICINAL & NON-MEDICINAL FOOD SUPPLEMENT PRODUCTS
SOLUBLE ORAL POWDERS (SOP)
Production equipment
3 mixers: 600 l, 2000 l, 3000 l
Packaging equipment
5 pieces of equipment for 10 g to 25 kg packages
Main types of packaging
-Sacks: 5 kg to 25 kg
-Pots: 50 g to 1 kg
-Buckets: 2 – 3 kg
MEDICATED PRE-MIXES (MP)
Production equipment
1 carousel of pre-mixes – Lot size 1000-2000 kg
Packaging equipment
1 bagging line
Main types of packaging
-Sacks: 5 kg to 25 kg
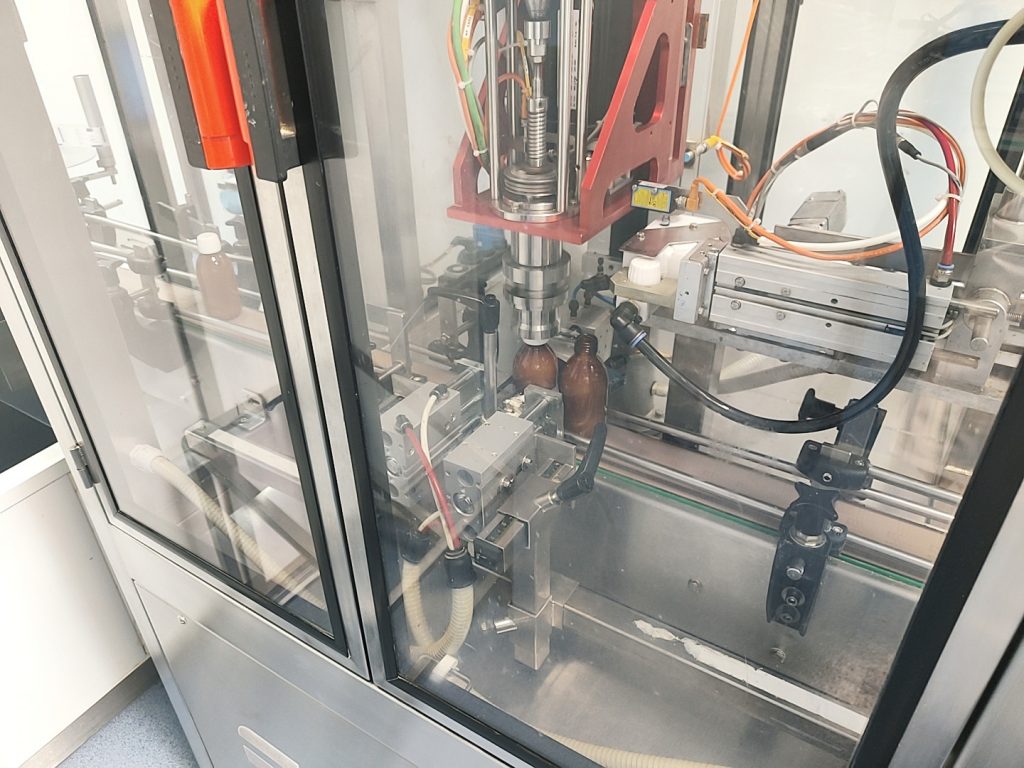
LIQUIDS (SOLUTIONS / SUSPENSION)
Production equipment
3 mixing vats: 1500 l to 4000 l
Packaging equipment
5 pieces of equipment for the main packages
Main types of packaging
-Sticks: 4 ml to 10 ml
-Bulk: IBC (intermediate bulk container) 1000 l
DOUGHS
Production equipment
1 mixing vat: 1000 l
Packaging equipment
1 piece of equipment for the main packages
Main types of packaging
Tubes: 300-400 g
INTRARUMINAL DEVICE
Production equipment
1 lamination cabin
Packaging equipment
1 Bolus Intra R equipment in tube
QUALITY CONTROL LABORATORY

Chromatography analysis unit
Principal analyses: Identification, dosages and search for related substances 3 liquid chromatography devices (HPLC) and 1 chromatography device in the gaseous phase (CPG)

Chemical physics analysis unit
Principal analyses: Dosages (potentiometry, UV spectrophotometry and burette) Identifications (thin-layer chromatography, IR spectroscopy, moisture content, refraction index, etc.) Techniques: Karl-Fisher, densitometry, pH-metry, spectrophotometry, etc.

Microbiological analysis unit
Principal analyses:
Dosage
Microbial contamination
Search for pathogens

Control unit of the Printed Packaging Items

Sample Library
- ANSES / GMP Agreement: V 0594/81
- DDPP Agreement: αFR95355023
- 897 891 370 – RCS Pontoise
- SIRET: 897 891 370 00024
- TVA Intracommunautaire: FR30897891370
- Manufacturing equipments
- 3 mixing tanks
- 600L, 2000L, 3000L
- Packaging equipments
- 5 primary packaging equipments
- 10g to 25kg
- Packaging: Sachets, bags, pots, buckets
- 3 mixing tanks
Non-pharmaceutical dedicated area
- Manufacturing equipments
- 1 mixing tanks
- 1000L
- Packaging equipments
- 1 primary packaging equipment
- Tubes: 300-400g
- 1 mixing tanks
- Manufacturing equipments
- FC France is a CDMO specialized in the development, manufacturing and packaging of veterinary product
- Non-sterile orale form
- Soluble oral powder form
- Liquid (soution/suspension) form
- Paste form
- Secondary packaging
- Manufacturing equipments
- 4 mixing tanks
- 1500L to 4000L
- Packaging equipments
- 5 primary packaging equipments
- Bottles: 50mL to 10L
- Sticks: 4-8mL
- 4 mixing tanks
- Carrying out checks on all the batches, ranging from the received raw materials to the finished product, in addition to the intermediate levels and packaging items.
- Monitoring the corresponding environmental conditions.
- Ensuring the specifications are met: raw materials, intermediate levels, finished products and packaging items.
- Updating techniques and regulations.
QC LABORATORY
- Raw material checks
- Over 100 products corresponding to more than 800 batches
- Analytical checks of the finished products
- Oral powders
- Liquids and pastes
- Premixes
- Packaging item (AC) and printed packaging item (ACI) checks
- Finished product and raw material sample library
QC PHYSCHEM LABORATORY
- Chromatography analysis unit
- Main analyses: Related substance identifications, assays and research
- 3 liquid chromatographs (HPLC) and
- 1 gas chromatograph (CPG)
- Chemical analysis unit
- Main analyses:
- Assays (potentiometry, UV spectrophotometry and burettes)
- Identifications (CCM, IR, water content, refractive index, etc.)
- Techniques: Karl Fischer, densimetry, pH-metry, spectrophotometry, etc.
- Main analyses:
MICROBIOLOGY QC LABORATORY
- Main analyses
- Assay
- Microbial contamination
- Pathogen research
Team
Friulchem S.P.A. – Disma Giovanni Mazzola
PRESIDENT
Biagio Giugliano
GENERAL MANAGER – COO & CFO
Pierre Reynaud De La Gardette
GENERAL MANAGER – OPERATION MANAGER
Marc Feron
QA MANAGER